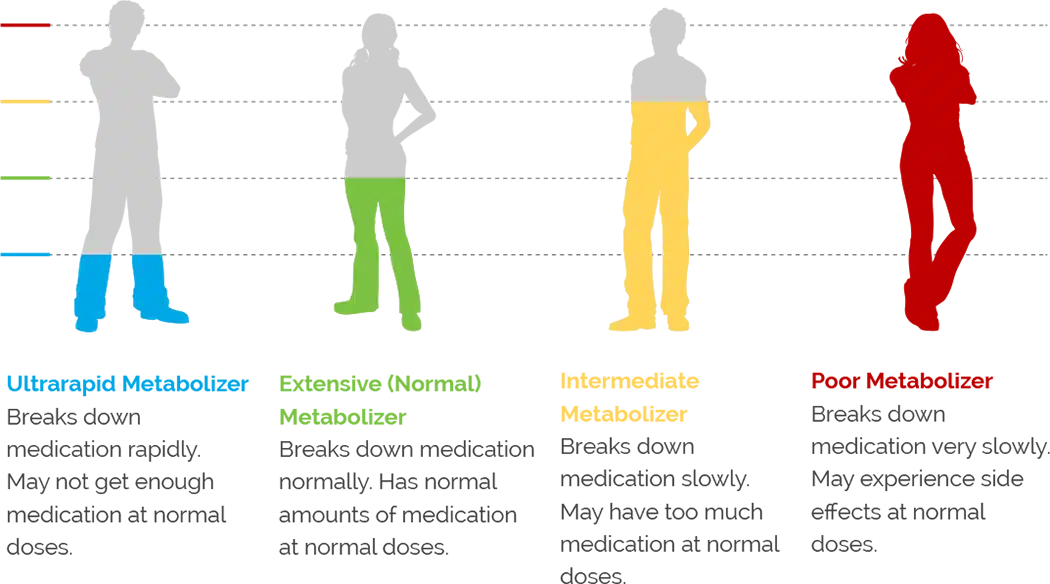
On the GeneSight Psychotropic Test, three color-coded categories are used to assist in report interpretation.
These medications are not associated with any known genetic issues that would be expected to change patient medication outcomes. However, these medications are not guaranteed to work and may not always be the best options, as there are many other factors that influence medication response and susceptibility to side effects, including drug-drug interactions, diet, environmental factors, age, etc.
These medications may require dose adjustments in order to have the desired effect, may be less likely to work, or may cause side effects.
These medications are likely to require dose adjustments in order to have the desired effect, may be less likely to work, or may cause side effects. Genetics are expected to have a greater impact on medications in the significant gene-drug interaction category than those that fall into the moderate gene-drug interaction category.
Many factors may influence medication outcomes in addition to genetics. Smoking status is one environmental factor that can contribute to a gene-drug-environment interaction. This is because smoking can increase the activity of CYP1A2 in patients who have the highly inducible CYP1A2 variant. For these patients, the categorization and information provided for certain medications can be affected by smoking status, where smoking is defined as the daily inhalation of burning plant material (cigarettes, marijuana), and excludes vaping and e-cigarettes.
For patients who carry the highly inducible CYP1A2 variant, the CYP1A2 phenotype is dependent on their smoking status.
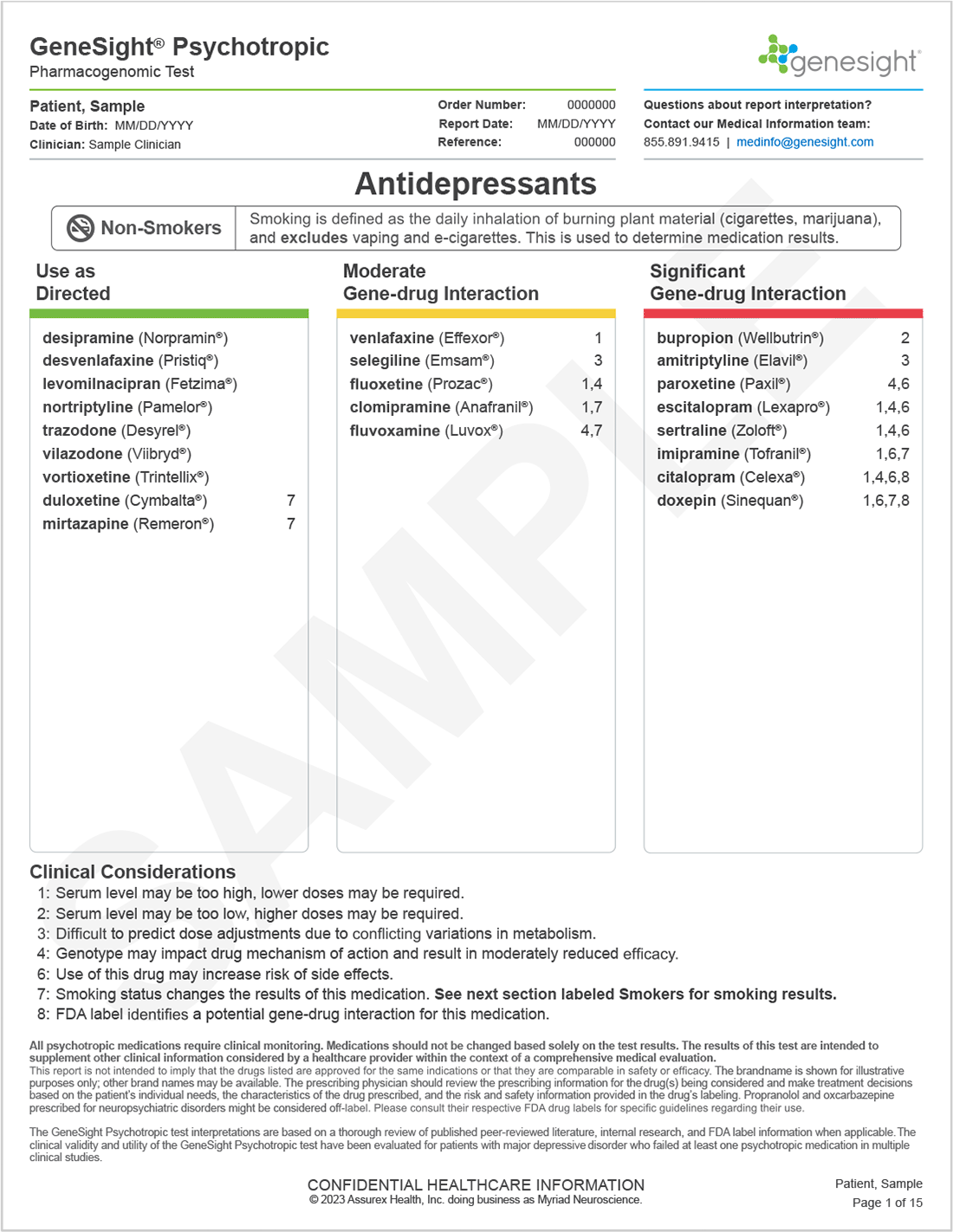
Non-smokers who have the highly inducible variant would be expected to have normal CYP1A2 enzyme activity, and the sections labeled Non-Smokers should be used.
Smokers with the highly inducible variant may have increased CYP1A2 enzyme activity, and the sections labeled Smokers should be used.
It is important to be aware of smoking status and refer to the correct sections of the report for individuals with the highly inducible CYP1A2 variant.
Some patients (approximately 9%) do not have the highly inducible variant found in CYP1A2.
For these individuals, their reports will note that the results provided are for both non-smokers and smokers since the individual does not have the highly inducible CYP1A2 variant. Therefore, smoking status does not have to be considered as it does for those with the highly inducible variant.
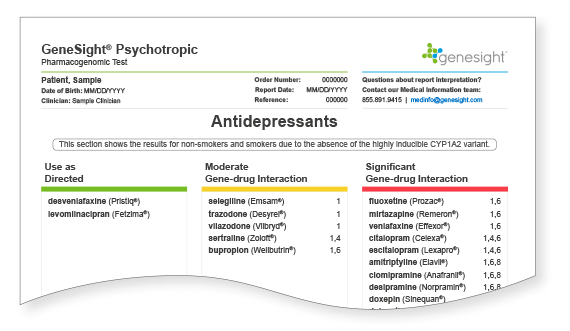
Interpreting the Clinical Considerations
All medications start in the “Use as Directed” category on the report. Based on an individual’s genetic variation, medications may be moved to the Moderate or Significant Gene-drug Interaction categories, depending on how significantly the variation is expected to impact outcomes with that medication. Medications in the Moderate or Significant Gene-drug interaction categories on the report do not necessarily need to be avoided. The clinical considerations, which are denoted by numbers next to the medications, explain the rationale for a medication’s classification and can be used to help inform treatment decisions.
Clinical Considerations
- Serum level may be too high, lower doses may be required.
- Serum level may be too low, higher doses may be required.
Clinical Considerations 1 and 2 reflect an issue with how a medication is metabolized, which means the individual has variation in one or more pharmacokinetic genes. This may affect how much medication is in an individual’s system and may require a lower or higher dose. For medications that may require a higher dose, it is not advising to start the individual on a high dose of the medication, but rather to start at a standard dose while being aware that dose increases may be needed to get the desired benefit.
- Difficult to predict dose adjustments due to conflicting variations in metabolism.
Clinical Consideration 3 also provides information about a medication’s metabolism. This clinical consideration indicates that there are conflicting variations in the metabolism of that medication, thus making it difficult to predict a dose adjustment. The most common example is when there are two genes that contribute a relatively similar amount to the overall metabolism of the medication, but one has increased activity and the other has decreased activity. If a medication with clinical consideration 3 is chosen, it may be appropriate to consider starting on a lower dose and increasing the dose based upon how the individual is doing.
- Genotype may impact drug mechanism of action and result in moderately reduced efficacy.
Clinical Consideration 4 indicates that an individual may have a moderately reduced response to certain medications. This is due to variation in a pharmacodynamic gene, and thus reflects an issue with the drug’s mechanism of action. Since pharmacodynamic genes do not affect metabolism, it is unlikely that adjusting the dose will improve efficacy.
- CYP2D6 genotype indicates that this patient may experience increased frequency of side effects but also greater symptom improvement in those who find the treatment tolerable.
Clinical Consideration 5 specifically applies to atomoxetine (Strattera®) when an individual is a CYP2D6 poor metabolizer. This is based on evidence which shows that CYP2D6 poor metabolizers treated with atomoxetine experienced an increased frequency of side effects but also significantly greater improvement in ADHD symptoms compared to extensive (normal) metabolizers.1
- Use of this drug may increase risk of side effects.
Clinical Consideration 6 indicates that an individual may have an increased risk for side effects when taking this medication. This can be due to variation in one or more pharmacokinetic genes that may predict slower than normal metabolism of certain medications, which may result in higher levels of medication in the system. It could also be due to an effect caused by one of the pharmacodynamic genes.
- Smoking status changes the results of this medication. See next section labeled Smokers for smoking results.
Clinical Consideration 7 specifically applies to individuals who have the highly inducible variant of the CYP1A2 gene, which may result in increased activity for CYP1A2 when the patient is a smoker. This increased activity may change how this medication is metabolized. For individuals who are smokers, it is important to refer to the results in the following section labeled Smokers.
- FDA label identifies a potential gene-drug interaction for this medication.
When medications have Clinical Consideration 8, it means that FDA label contains information that may be relevant for the patient based on their genetic results. This may include information about dosing or more general information to consider.
- Per FDA label, this medication is contraindicated for this genotype.
Clinical Consideration 9 indicates that per the FDA label, this medication is contraindicated for an individual with this genotype.
- While this medication does not have clinically proven genetic markers that allow it to be categorized, it may be an effective choice based on other clinical factors.
Clinical Consideration 10 is associated with medications in the gray “No Proven Genetic Markers” category, meaning that genetic markers have not yet been discovered to reliably predict treatment outcomes with these medications. Due to this lack of evidence, we are not currently able to categorize them or provide actionable recommendations. However, these medications may be effective choices based on other clinical factors. Medications hat fall into the “No Proven Genetic Markers” category and receive a clinical consideration of 10 are the same for all individuals.
Interpreting the Patient Genotypes and Phenotypes
The patient genotypes and phenotypes section of the report provides a list of all the genes tested on the GeneSight® Psychotropic report along with the individual’s specific genetic result (genotype) and the characteristic associated with the genetic result (phenotype). The patient genotypes and phenotypes are broken down into two categories:
Pharmacodynamic genes provide information about how a medication works on the body. Variation in these genes may affect likelihood of response or risk of side effects with certain medications.
ADRA2A
ADRA2A encodes the alpha-2A adrenergic receptor, which is a norepinephrine receptor. Individuals with the C/C genotype may have a moderately reduced response to certain stimulants, specifically methylphenidate and dexmethylphenidate. If an individual with this genotype is having a less than optimal response to these medications, increasing the dose may not necessarily improve efficacy.
HLA-A*3101
The human leukocyte antigen (HLA) complex, encoded by the HLA gene family, plays a critical role in immunity. Presence of the T allele (either T/T or A/T genotype) is associated with a higher risk of serious hypersensitivity reactions, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), maculopapular eruptions, and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), when taking certain mood stabilizers, specifically carbamazepine.
HLA-B*1502
Presence of genetic variation in HLA-B*1502 is associated with a higher risk of serious dermatologic reactions, including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), when taking certain mood stabilizers, including carbamazepine, oxcarbazepine, and lamotrigine.
HTR2A
HTR2A encodes for the Serotonin Receptor Type 2A, which is responsible for serotonin signaling. Studies show an increased risk of adverse effects with certain SSRIs, particularly paroxetine, in individuals who have the G/G genotype.
SLC6A4
SLC6A4 encodes for the serotonin transporter, which is the main site of action for SSRIs. SLC6A4 has two main versions: the long (L) allele and the short (S) allele. Studies have shown that the short allele results in less serotonin transporters than the long allele. Individuals who have the S allele may be less likely to respond to certain SSRIs based on this genotype. Additionally, due to the lower number of transporters, increasing the dose may not necessarily improve efficacy.
Pharmacokinetic genes provide information about how the body works on the medication. Variation in these genes may affect the metabolism of a medication. The following PK genes are included on the GeneSight test: CES1A1, CYP1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, UGT1A4, and UGT2B15.
These genes are categorized based on average enzyme activity into one of four metabolizer phenotypes on the GeneSight Psychotropic report.
The GeneSight test uses a combinatorial approach that measures multiple genomic variants for each individual and weighs them in combination in order to categorize the medications on the report and provide clinical considerations when applicable.
The genotype for COMT, or catechol-o-methyltransferase, is provided for informational purposes only. This gene has not been shown to be a reliable marker of medication outcomes, and therefore, it is not used to categorize medications on the report.
Interpreting the Gene-drug Interaction Chart
The gene-drug interaction chart provides supplementary information about which pharmacokinetic genes are involved in the metabolism of each medication.
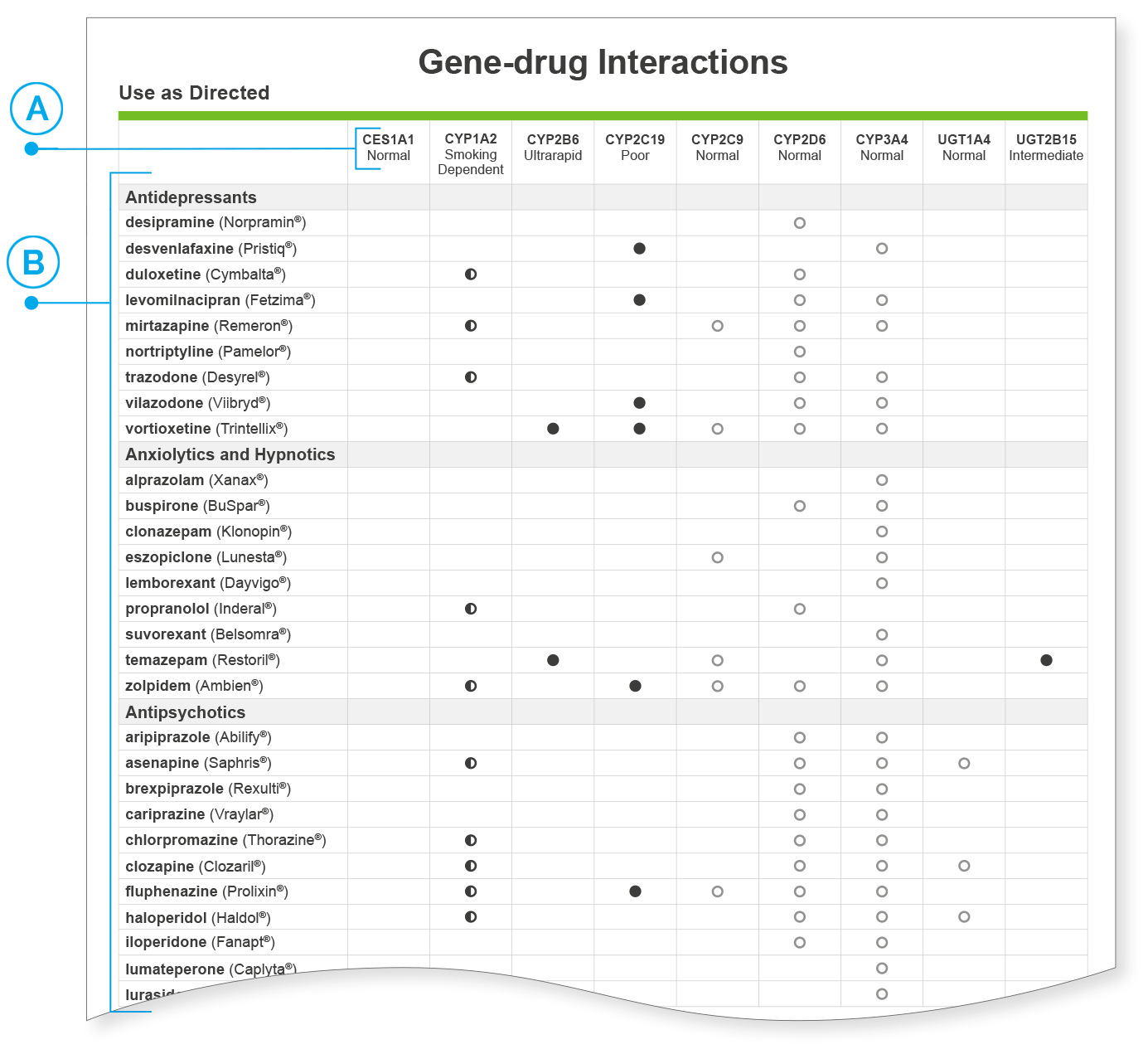
| The pharmacokinetic genes on the GeneSight® report are listed across the top of the chart. | |
| Medications are listed in a column on the left side. | |
| Any dot (either shaded, unshaded, or half-shaded) signifies that the enzyme is involved in the metabolism of the associated medication. | |
| A shaded dot means that variation was found in the patient’s genotype that may impact medication metabolism. | |
| An unshaded dot indicates that the gene is associated with medication metabolism, but the predicted patient phenotype is normal. | |
| A half-shaded dot means that the phenotype is dependent upon smoking status, with smokers being ultrarapid metabolizers and non-smokers being normal metabolizers. Medication categorization in this chart is according to non-smokers. Refer to sections labeled Smokers to see medication categories for individuals who smoke. |
It is not mandatory to refer to the gene-drug interaction chart when using the GeneSight test to inform patient medication selection. It is simply intended to augment the patient results on the pages where the medications are categorized and the clinical considerations are provided, which serve as the primary resource to help inform treatment decisions.
The format of the gene-drug interaction chart is similar to the earlier pages of the GeneSight report where medications are categorized into the green, yellow, and red categories.
There are situations where an individual may have shaded dots in the green “Use as Directed” category. This means that although the individual has variation in one or more genes, it is unlikely to impact their overall metabolism of that particular medication. This could mean that while an enzyme is involved in the metabolism of the medication, its role is not clinically significant enough to warrant a change in dosing or there may be compensation among the enzymes known to metabolize the medication, which results in the medication not being moved from the green “Use as Directed” category.
Frequently Asked Questions
A medication falls in the green category when no variation was found in the genes tested by the GeneSight® test that is expected to impact how a patient may break down or respond to that medication. However, medications that fall in the green category may not always work for a patient. Pharmacogenomics identifies potential genetically related issues with medications (i.e., those that fall in the yellow or red category); it does not identify medications that will work. Additionally, while genetics provides an important piece of the puzzle, there are many factors that can influence medication response and susceptibility to side effects other than the genes that are tested on the GeneSight panel, including drug-drug interactions, diet, psychological factors, environmental factors, age, etc. Genetics can be a helpful tool, but it is important to take into consideration a patient’s entire clinical profile for medication selection.
Medications are listed in alphanumeric order. Those with the fewest numbers, or clinical considerations, are placed at the top of the list in alphabetical order, and the medications with more clinical considerations are placed further down the list. It is important to consider what the clinical considerations are saying as they may help inform on medication selection and treatment decisions for medications affected by gene-drug interactions.
While clinical studies have shown that patients on red category medications had less improvement in depression treatment outcomes, this may not be the case for all patients who undergo GeneSight testing. It is important to acknowledge that medication response is multifactorial, and genetics is one factor that may influence how a medication works alongside other non-genetic factors such as drug-drug interactions, diet, psychological factors, environmental factors, age, etc. GeneSight test results are intended to supplement other clinical and patient specific factors to help inform medication selection and treatment decisions.
Medications are categorized by the GeneSight algorithm based on the accumulation of genetic issues (gene-drug interactions) detected. The GeneSight test takes into account all relevant genes known to affect metabolism or response to each medication and the relative influence of each gene. Thus, a medication in the red category has greater cumulative genetic issues than a medication in the yellow category, and this may be because different genes are involved in predicting changes in metabolism or response to it, or because the same gene plays a larger role in the red category medication than in the yellow category medication.
An all-green report indicates that no significant variation was detected in the patient’s genes tested by the GeneSight test that would be expected to affect their outcomes with medications on the GeneSight report. This occurs <1% of the time for GeneSight Psychotropic. An all-green report may be useful in helping ‘rule out’ genetic causes of medication intolerance or lack of response, and may lead to alternative treatment approaches, such as psychotherapy.
From a genetic perspective, the genetic results shown on the GeneSight report will be accurate regardless of what medications a patient is taking. The GeneSight test evaluates only gene-drug interactions. In other words, it looks at potential changes in a patient’s DNA to predict how this might alter the patient’s expected outcomes with certain medications. Since a person’s genes will not change based on what medications they are taking, the GeneSight results will not change. However, it is possible for certain medications to interact with medications on the report, and this is called a drug-drug interaction. Drug-drug interaction potential should be taken into consideration alongside GeneSight test results as they may affect medication outcomes in addition to the genetic information provided on the GeneSight report.
The results of the GeneSight test are intended to supplement other clinical factors considered by a healthcare provider during a comprehensive medical assessment to help inform treatment decisions.
Not all patients who receive the GeneSight test will have improved outcomes. The GeneSight test is intended to supplement a clinicians comprehensive medical assessment.
- Michelson D, et al. 2007. J Am Acad Child Adolesc Psychiatry.