What is CYP1A2?
CYP1A2 is part of the drug-metabolizing enzyme family, known as cytochrome P450. CYP1A2 plays an important role in the metabolism of several mental health medications, including duloxetine, fluvoxamine, clozapine, olanzapine, and mirtazapine. It is involved in metabolizing around 36% of medications on the GeneSight® Psychotropic report. One of the most studied polymorphisms in the CYP1A2 gene is a C to A nucleotide switch at position -163 within intron 1 (-163C>A).1 The A allele has been shown to be highly inducible by smoking, causing increased metabolic activity.2
How does smoking induce the CYP1A2 enzyme?
The aryl hydrocarbon receptor (AHR) plays an important role in the regulation of CYP1A2. AHRs are activated by the binding of polycyclic aromatic hydrocarbons (PAHs) that are produced through the burning of tobacco. This activation leads to the enhanced expression of the CYP1A2 gene.3-5
How is smoking status defined?
Smoking is defined as the daily inhalation of burning plant material (cigarettes, marijuana). This excludes vaping and e-cigarettes since the induction is driven by the inhalation of hydrocarbons that are produced by the burning of plant material, rather than by nicotine.6-8 Although most studies have evaluated the effect of cigarette smoking on CYP1A2 activity, interactions between marijuana smoking and the metabolism of CYP1A2 substrates have been observed and are expected to be mechanistically similar to the interactions between tobacco smoking, CYP1A2 substrates, and the CYP1A2 -163C>A variant.9-13
What do the data say about the impact of the CYP1A2 -163C>A polymorphism and smoking status on CYP1A2 substrates?
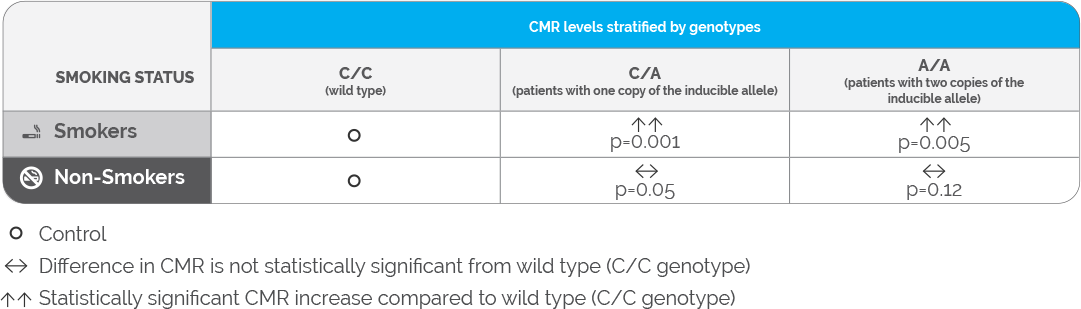
A meta-analysis by Koonrungsesomboon et al. assessed the effects of genetic polymorphisms on CYP1A2 activity in 1,662 healthy subjects.14 The authors observed that individuals who were homozygous (A/A) or heterozygous (C/A) for the CYP1A2 −163C>A polymorphism had higher caffeine metabolic ratios (CMRs) compared to wild-type individuals (homozygous: p = 0.005; heterozygous: p = 0.003). However, after segregating these 8 studies into smokers and non-smokers, the differences in the CMRs were only statistically significant in the smoking cohort (Table 1). These results suggest that the CYP1A2 promotor polymorphism may be clinically relevant only among individuals who are smokers, and the effect of this polymorphism in non-smokers may not be clinically meaningful.
Table 1: Summary of the meta-analysis assessing the effect of the CYP1A2 -163C>A polymorphism on CYP1A2 enzyme activity
Another meta-analysis by Takuathung et al.15 assessed the effect of the CYP1A2 -163C>A polymorphism on the pharmacokinetics of clozapine (n=327) and olanzapine (n=257) and did not find any differences in the plasma/serum levels of the medications between individuals who carried the inducible allele and wild type individuals. However, the analysis could not study the effect of smoking on this polymorphism as the studies included could not be segregated by smoking status. Nevertheless, the impact of the CYP1A2 promotor polymorphism was not seen when smoking status was not considered.
How is smoking status expected to impact the metabolism of certain mental health medications?
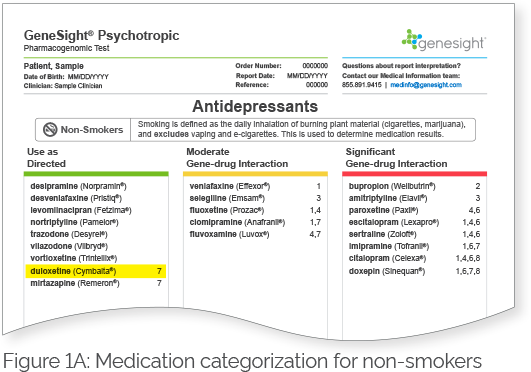
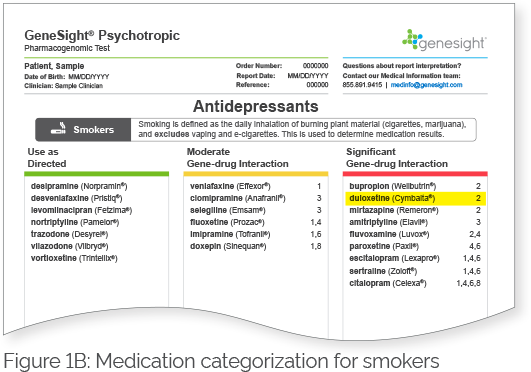
Patients who do not carry the highly inducible CYP1A2 variant (i.e., patients with the C/C genotype) are classified as CYP1A2 extensive (normal) metabolizers regardless of their smoking status (Table 2). The CYP1A2 phenotype for patients who carry the highly inducible CYP1A2 variant (i.e., patients with either the C/A or A/A genotypes) would be dependent on their smoking status. Smokers who have the highly inducible A allele would be considered ultrarapid metabolizers, whereas non-smokers who have the highly inducible A allele would be considered extensive (normal) metabolizers (Table 2). Therefore, smokers and non-smokers may break down certain mental health medications that are substrates of CYP1A2 differently. These medications will be labeled with a clinical consideration 7 on the GeneSight Psychotropic report, which will alert the clinician and patient that smoking status changes the results of the medication, and the results in the next section labeled Smokers should be used for individuals who smoke. For example, Figure 1A shows the results for non-smokers, and duloxetine is in the green category with clinical consideration 7. Based on this sample patient’s genetic results, if they were a non-smoker, they would be expected to break down duloxetine normally. However, as indicated by clinical consideration 7, smoking status changes the results of this medication, and the results in the next section labeled Smokers should be used for individuals who smoke. Figure 1B shows the results for smokers, and duloxetine is in the red category with clinical consideration 2. This indicates that based on this sample patient’s genetic results, if they were a smoker, they would be expected to break down duloxetine more quickly than normal. Therefore, it is important to take smoking status into consideration in patients with the C/A or A/A genotypes when determining their CYP1A2 phenotype. Patients with the C/C genotype would not have medications segregated for smokers and non-smokers as they do not have the highly inducible allele.
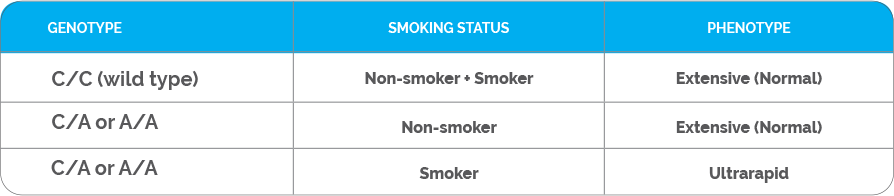
Figure 1. GeneSight Psychotropic sample report showing the impact of smoking status on medication categorization for patients with the highly inducible CYP1A2 allele.
For more information, contact the Medical Information Department at:
PHONE: 855.891.9415
EMAIL: medinfo@assurexhealth.com
References
- The Pharmacogene Variation (PharmVar) Consortium- CYP1A2 https://www.pharmvar.org/gene/CYP1A2. (2017).
- MacLeod, S. L., Tang, Y.M., et al. The role of a recently discovered genetic polymorphism in the regulation of the human CYP1A2 gene. Proceedings of the American Association for Cancer Research 39, 396 (1998).
- Zhou, S. F., Yang, L. P., Zhou, Z. W., Liu, Y. H. & Chan, E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. Aaps j 11, 481-494 (2009). https://doi.org/10.1208/s12248-009-9127-y
- Zevin, S. & Benowitz, N. L. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet 36, 425-438 (1999). https://doi.org/10.2165/00003088-199936060-00004
- Wang, Q., VonHandorf, A. & Puga, A. in Encyclopedia of Signaling Molecules (ed Sangdun Choi) 1-15 (Springer New York, 2016).
- van der Plas, A. et al. Impact of switching to a heat-not-burn tobacco product on CYP1A2 activity. Toxicology Reports 7, 1480-1486 (2020). https://doi.org/10.1016/j.toxrep.2020.10.017
- Hukkanen, J., Jacob, P., 3rd, Peng, M., Dempsey, D. & Benowitz, N. L. Effect of nicotine on cytochrome P450 1A2 activity. Br J Clin Pharmacol 72, 836-838 (2011). https://doi.org/10.1111/j.1365-2125.2011.04023.x
- Valodia, P. N. The role of heat-not-burn, snus and other nicotine-containing products as interventions for epileptic patients who take phenytoin and smoke cigarettes. Toxicology Reports 9, 1114-1119 (2022). https://doi.org/10.1016/j.toxrep.2022.03.050
- Chetty, M., Miller, R. & Moodley, S. V. Smoking and body weight influence the clearance of chlorpromazine. Eur J Clin Pharmacol 46, 523-526 (1994). https://doi.org/10.1007/BF00196109
- Jusko, W. J. et al. Factors affecting theophylline clearances: age, tobacco, marijuana, cirrhosis, congestive heart failure, obesity, oral contraceptives, benzodiazepines, barbiturates, and ethanol. J Pharm Sci 68, 1358-1366 (1979). https://doi.org/10.1002/jps.2600681106
- Jusko, W. J., Schentag, J. J., Clark, J. H., Gardner, M. & Yurchak, A. M. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther 24, 405-410 (1978).
- Anderson, G. D. & Chan, L. N. Pharmacokinetic Drug Interactions with Tobacco, Cannabinoids and Smoking Cessation Products. Clin Pharmacokinet 55, 1353-1368 (2016). https://doi.org/10.1007/s40262-016-0400-9
- Stout, S. M. & Cimino, N. M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metabolism Reviews 46, 86-95 (2014). https://doi.org/10.3109/03602532.2013.849268
- Koonrungsesomboon, N., Khatsri, R., Wongchompoo, P. & Teekachunhatean, S. The impact of genetic polymorphisms on CYP1A2 activity in humans: a systematic review and meta-analysis. Pharmacogenomics J 18, 760-768 (2018). https://doi.org/10.1038/s41397-017-0011-3
- Na Takuathung, M., Hanprasertpong, N., Teekachunhatean, S. & Koonrungsesomboon, N. Impact of CYP1A2 genetic polymorphisms on pharmacokinetics of antipsychotic drugs: a systematic review and meta-analysis. Acta Psychiatr Scand 139, 15-25 (2019). https://doi.org/10.1111/acps.12947

