A medication falls in the green category when there is no variation found in the patient’s genes that is expected to impact their outcomes with that medication. In other words, the patient’s phenotype is “normal” for all genes that are known to interact with a particular medication. When all medications fall in the green “Use as Directed” category, it means the patient did not have any significant variation in any of the genes that are tested on the GeneSight® test. This occurs <1% of the time for GeneSight Psychotropic.
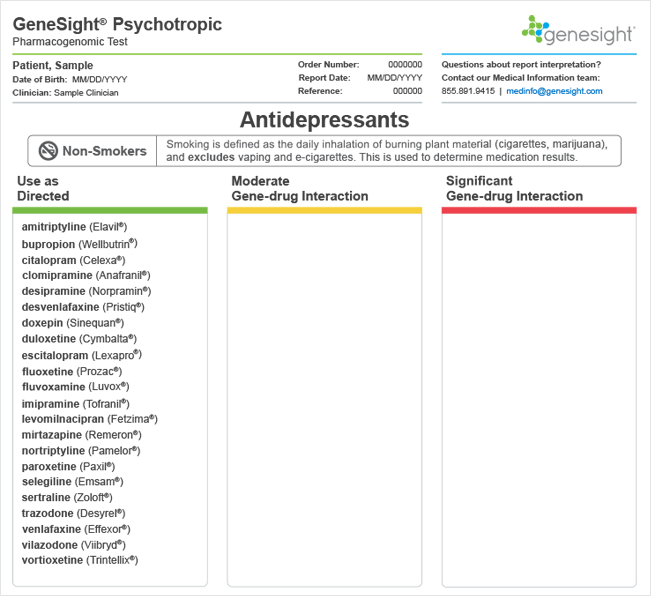
Patients who were unknowingly prescribed medications in their green category on the GeneSight test had better outcomes compared to patients who were unknowingly prescribed medications in their red category.1-4 However, patients may not always respond to medications that fall in their green category. There are many factors that influence patient response and susceptibility to side effects other than the genes that are tested on the GeneSight test. These include drug-drug interactions, diet, social/contextual factors, psychological factors, environmental factors, age, or gender. Furthermore, as more is learned about the genetics and biology of mental illness and its treatment, additional variants may be added to the GeneSight panel, as well as additional medications with different mechanisms of action that may better target specific biological subtypes of depression.
Is an all green report clinically useful?
An all green report does provide important clinical information. It can help ‘rule out’ a genetic problem contributing to the patient’s morbidity—similar to the way a normal CBC, BMP, or TSH might help ‘rule out’ a medical contribution to the illness.
Since an all green report decreases the odds that the genes tested for are at the root of the patient failing to respond or experiencing adverse effects, it may direct healthcare providers to consider alternative explanations for medication failure (i.e. drug-food interactions, adherence, comorbid conditions, drug-drug interactions, etc.). For some patients, this can lead to alternate approaches to treatment. For example, one published case study describes a patient who reported experiencing side effects to several antidepressants. Most medications on this patient’s report were in the green category, and genetic variation could not explain the patient’s acute sensitivity to various medications. However, the report did lead the healthcare provider to look into the psychological aspects of the patient’s sensitivity and to treat the patient’s somatic obsessions.5 Patients who have failed multiple medication trials and are found not to have genetic variation supporting poor medication response may benefit from a non-pharmacologic intervention or augmentation such as psychotherapy, TMS, ECT, or DBS.
References
- Winner, J. G., Carhart, J. M., Altar, C. A., Allen, J. D., & Dechairo, B. M. (2013). A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discovery Medicine, 16(89), 219–27.
- Hall-Flavin, D. K., Winner, J. G., Allen, J. D., Carhart, J. M., Proctor, B., Snyder, K. A, … Mrazek, D. A. (2013). Utility of integrated pharmacogenomic testing to support the treatment of major depressive disorder in a psychiatric outpatient setting. Pharmacogenetics and Genomics, 23(10), 535–48.
- Altar, C.A., Carhart, J., Allen, J.D., Hall-Flavin, D., Winner, J., Dechairo, B. (2015). Clinical utility of combinatorial pharmacogenomics-guided antidepressant therapy: evidence from three clinical studies. Molecular Neuropsychiatry, 1(3), 145-155.
- Altar, C.A., Carhart, J.M., Allen, J.D., Hall-Flavin, D.K., Dechairo, B.M., Winner, J.G. (2015). Clinical validity: Combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. The Pharmacogenomics journal, 15(5), 443-451.
- Winner, J.G., Allen, J.D., Lorenz, J.P., Altar, C.A. (2012). Overwhelmed by side effects. Curr Psychiatr., 11(6).

