Clinical Information for GeneSight® Psychotropic
Summary of Clinical Studies
The clinical validity, clinical utility and economic utility of the GeneSight Psychotropic test have been evaluated in multiple peer-reviewed publications. In fact, it’s the only neuropsychiatric pharmacogenomic test backed by such extensive research. It is important to note that not all patients who received a GeneSight Psychotropic test experienced improved outcomes. However, overall, GeneSight Psychotropic helped improve outcomes compared to treatment as usual in the following studies.
Study Design: This was an 8 week, blinded, multi-center, randomized controlled trial of 1,167 subjects with major depressive disorder from 20 academic sites and 40 community sites. The trial was unblinded after the 8 week check-in, and the subjects were followed out to 24 weeks. The study assessed the impact of the GeneSight Psychotropic test on psychiatric treatment response compared to treatment as usual (TAU). The raters used the Hamilton Rating Scale for Depression (HAM-D17), and the subjects had to have a minimum score of 14 in order to be eligible for the study.
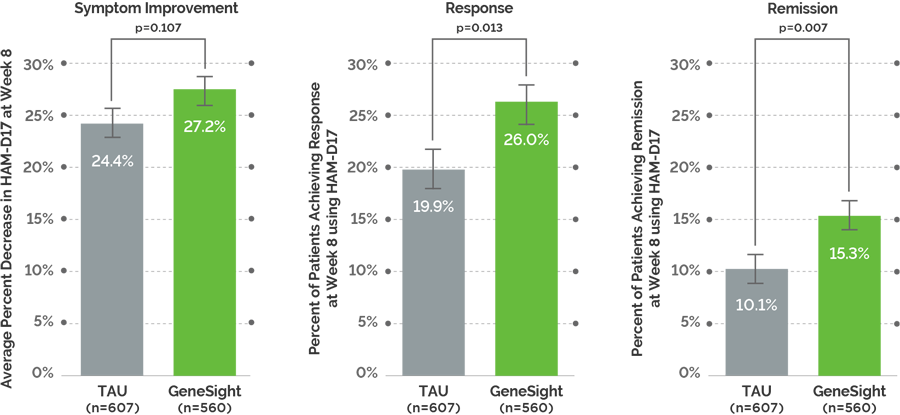
Study Endpoints: GUIDED compared two active treatment arms. The primary endpoint was symptom improvement with secondary endpoints of response and remission. Symptom improvement is defined as the change in HAM-D17 score, and this is based on the group average. Response is defined as a ≥50% reduction in HAM-D17 score, and remission is defined as a HAM-D17 score ≤7.
Study Limitations: The treating clinician was not blinded to study arm, the majority of the cohort was Caucasian, the primary results may not be generalizable for patients with mild depression, and the impact of polypharmacy on patient outcomes was not evaluated.
Key Findings
Directional improvement in symptoms: On the primary endpoint of symptom improvement, the data trended toward but did not achieve statistical significance between GeneSight and TAU arms at week 8.
Significant improvement in response and remission: There were statistically significant and clinically meaningful increases in response and remission rates in the GeneSight arm versus the TAU arm at week 8.
The GeneSight effect on all endpoints was durable over 6 months: Symptom improvement, response, and remission continued to improve after unblinding at week 8 and up to 6 months.
Switching to a medication with no or moderate gene drug interaction improved patient outcomes: Symptom improvement, response, and remission were significantly improved when patients on a medication with significant gene-drug interactions were switched to a medication with no or moderate gene-drug interactions by week 8 compared to those who remained on medications with significant gene-drug interactions.
Study Design: This was a prospective, open-label study of 165 subjects with a primary diagnosis of major depressive disorder. Subjects had to have a minimum score of 14 on the 17-item Hamilton Rating Scale for Depression (HAM-D17). Subjects were also assessed using the Quick Inventory of Depressive Symptomatology-Clinician Rated (QIDS-C16) and the Patient Health Questionnaire (PHQ-9). The study compared patient outcomes between GeneSight group and treatment as usual group (TAU) at week 8.
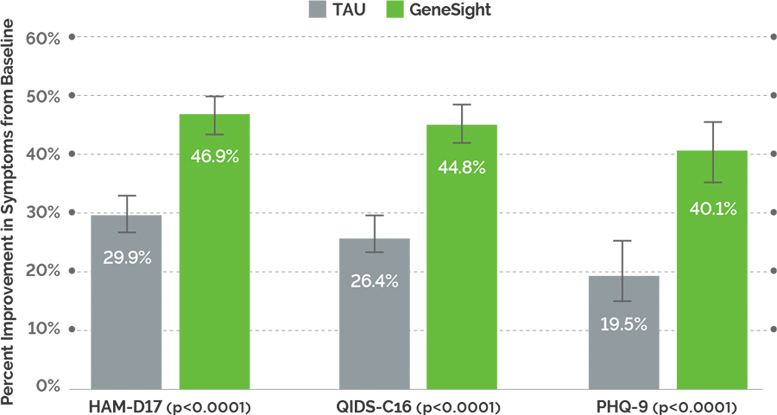
Study Endpoints: Symptom improvement, response and remission were measured across the three scales. Symptom improvement is defined as the change in the scale score, and this is based on the group average. Response is defined as a ≥50% reduction in the scale score. Remission is defined as a HAM-D17 score ≤7, a QIDS score <6,, or a PHQ-9 score <5.
Study Limitations: This study had a relatively small sample size and was not blinded.
Key Findings
Significant improvement in depression scores: Greater reduction in depression ratings was observed across the duration of the study for the GeneSight group, showing 70% greater improvement in depressive symptoms compared to TAU at 8 weeks (QIDS-C16).
Higher response rates: Patients in the GeneSight group were 2.1 times more likely to respond to their medications compared to those in the TAU group.
Higher physician and patient satisfaction: Almost three times as many physicians in the GeneSight group perceived their patients to be very highly satisfied with their care compared to the TAU group. Physician reporting of confidence in choice of medication and treatment and satisfaction with care was also substantially higher in the GeneSight group.
Study Design: This was a blinded, randomized controlled trial of 49 subjects with a primary diagnosis of major depressive disorder. Subjects had to have a minimum score of 14 on the 17-item Hamilton Rating Scale for Depression (HAM-D17). It compared patient outcomes between the GeneSight group and the treatment as usual group (TAU) at week 10.
Study Endpoints: Symptom improvement, response, and remission were measured using HAM-D17. Symptom improvement is defined as the change in HAM-D17 score, and this is based on the group average. Response is defined as a ≥50% reduction in HAM-D17 score, and remission is defined as a HAM-D17 score ≤7.
Study Limitations: The treating clinician was not blinded to study arm, the study was underpowered, and the results were not statistically significant.
Key Findings
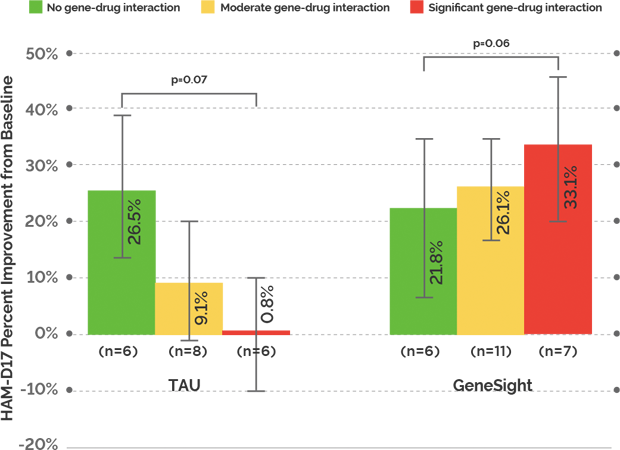
GeneSight accurately predicted patient outcomes based on gene-drug interactions: GeneSight accurately predicted those patients who were more likely to have poor depression outcomes due to gene-drug interactions. TAU subjects who had been prescribed medications with significant gene-drug interactions had almost no improvement in depressive symptoms over the 10 weeks of the trial.
Medications with no or moderate gene-drug interactions improved patient outcomes: When subjects who had been on medications with significant gene-drug interactions were switched to medications with no or moderate gene-drug interactions based on their GeneSight report, the subjects experienced a 33.1% improvement in symptoms (HAM-D17) at ten weeks compared to TAU subjects taking medications with significant gene-drug interactions, who had 0.8% improvement.
Study Design: This was a prospective,open-label study of 44 subjects with a primary diagnosis of major depressive disorder. Subjects had to have a minimum score of 14 on the 17-item Hamilton Rating Scale for Depression (HAM-D17). Subjects were also assessed using the Quick Inventory of Depressive Symptomatology-Clinician Rated (QIDS-C16). The study compared patient outcomes between the GeneSight group and the treatment as usual group at week 8.
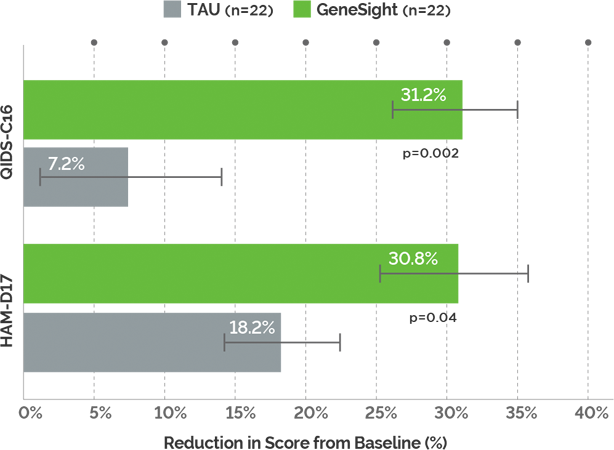
Study Endpoints: Symptom improvement was measured using HAM-D17 and QIDS-C16. Symptom improvement is defined as the change in the scale score.
Study Limitations: This study had a small sample size and was not blinded.
Key Findings
Greater Symptom Improvement: Patients in the GeneSight group experienced a 4-fold greater improvement in depressive symptoms at week 8 (QIDS-C16) compared to those in the treatment as usual (TAU) group.
With GeneSight, clinicians prescribed medications with no to moderate gene-drug interactions more often: Patients in the GeneSight group were more often prescribed medications with no to moderate gene-drug interactions vs. medications with significant gene-drug interactions.
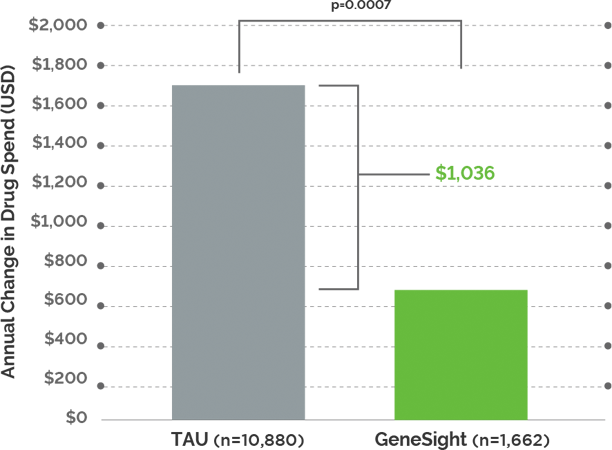
Study Design: Pharmacy claims were compared over one year between a cohort of GeneSight tested subjects (n = 2,168 enrolled) and a control group (n = 10,880). Both groups were followed for 365 days after the date of project enrollment. Prescription medication claims data was analyzed for differences between the two groups and within the GeneSight group based on medication changes that were congruent (medications with no or moderate gene-drug interactions) or non-congruent (medications with significant gene-drug interactions) with each individual’s GeneSight results.
Study Endpoints: Total pharmacy spend per member per year, adherence, discontinuation, and polypharmacy were measured.
Study Limitations: Study was not designed to evaluate efficacy of the GeneSight test. Cost savings is not directly associated with efficacy, and the results cannot be interpreted to be an indicator of efficacy.
Key Findings
Significant annual savings were realized for patients in the GeneSight group: Patients who received GeneSight testing saved, on average, $1,035.60 in total annual medication costs compared to TAU patients. Of that total, annual medication savings were $714.24 for non-CNS medications and $321.36 for CNS medications.
Improvement in Adherence: An increase in medication adherence of 17% was observed in the GeneSight group (p < 0.0001) for the medication prescribed after enrollment compared to the medication prescribed prior to enrollment.
Reduced Polypharmacy: One in five patients in the GeneSight group were on fewer medications by the end of the study (significantly greater than the TAU cohort, p < 0.0001).
Taking medications with no or moderate gene-drug interactions resulted in lower medication costs: GeneSight tested patients with congruent medication regimens saw an average of $2,774.53 in net annual cost savings compared to patients with incongruent medication regimens.
Worked across Practice Settings: Both psychiatrists and non-psychiatrists who had access to GeneSight information saw average savings of over $1,000 per patient.
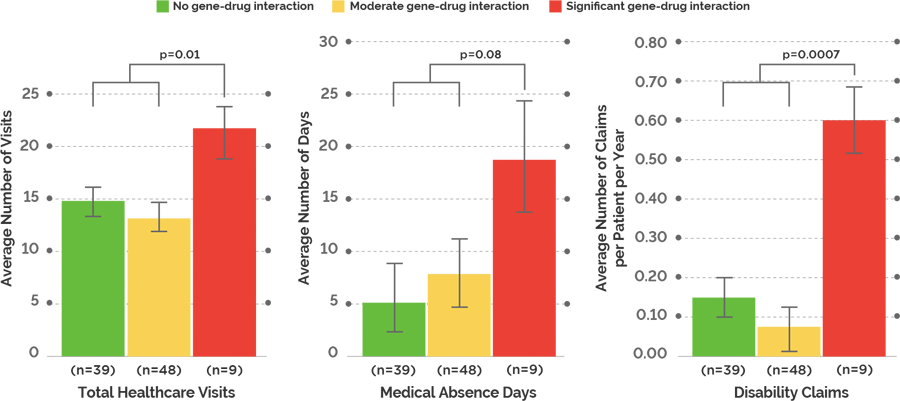
Study Design: The effect on healthcare utilization when prescribed a medication with significant gene-drug interactions was evaluated in a 1-year, blinded, retrospective study of 96 subjects with a DSM-IV-TR diagnosis of depression or anxiety disorder.
Study Endpoints: Healthcare visits, medical visits, psychiatric visits, medical absence days, disability claims, and healthcare spend were measured. Healthcare utilization costs included medical visit costs, psychiatric visit costs, ER costs, hospital costs, prescription costs, medical absence costs, and disability claim costs. Total healthcare visits included the total number of medical visits, psychiatrist visits, and ER visits.
Study Limitations: This study was retrospective, had a relatively small sample size, and included multiple diagnoses.
Key Findings
Taking medications with significant gene-drug interactions resulted in more total healthcare visits and disability claims: Patients taking medications with significant gene-drug interactions had nearly twice the number of total healthcare visits and about four times the number of disability claims compared to patients taking medications with no or moderate gene-drug interactions.
Employee productivity decreased when patients were on medications with significant gene-drug interactions: There is a 3-fold increase in medical absence days between patients on a medication with significant gene-drug interactions and those on a medication with no or moderate gene-drug interactions.
Switching patients to medications with no or moderate gene-drug interactions may decrease healthcare costs: When subjects were taking medications with significant gene-drug interactions, their average healthcare utilization cost was $8,627. In comparison, the average healthcare utilization cost was $3,453 for subjects taking medications with no gene-drug interactions and $3,426 for subjects taking medications with moderate gene-drug interactions. This resulted in an average annual increase in healthcare utilization cost of $5,188 for subjects taking medications with significant gene-drug interactions.
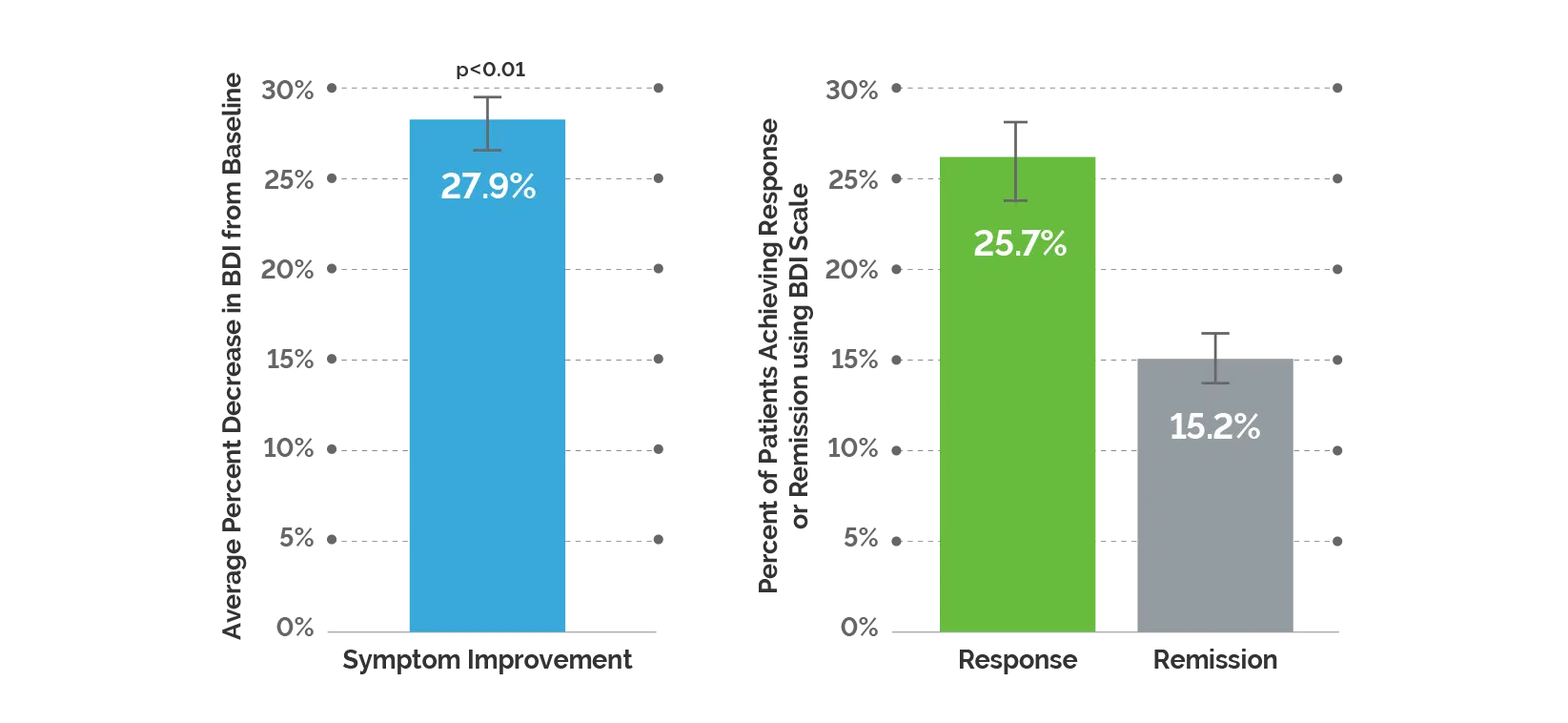
Study Design: This was an 8-12 week, prospective, naturalistic study that assessed 1,871 patients who had moderate-to-severe depression and were treated by primary care providers or psychiatrists. The study assessed the impact of the GeneSight Psychotropic test on patient outcomes using the Beck Depression Inventory. Patients had to have a BDI score ≥20 to be included in the study.
Study Endpoints: Symptom improvement, response and remission were measured using the BDI scale. Symptom improvement is defined by the percent change in BDI score. Response is defined as a ≥50% decrease in BDI score and remission is defined by a BDI score ≤10.
Study Limitations: This study did not have a control arm and was not blinded.
Key Findings
Significant improvement in depressive symptoms: Patients who received the GeneSight test experienced a 27.9% reduction in depressive symptoms at follow-up compared to baseline.
Significantly greater improvements among patients treated by PCPs: Symptom improvement, response, and remission were significantly better among patients treated by primary care providers (n=810) compared to psychiatrists (n=1,061).

